Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature
Abstract
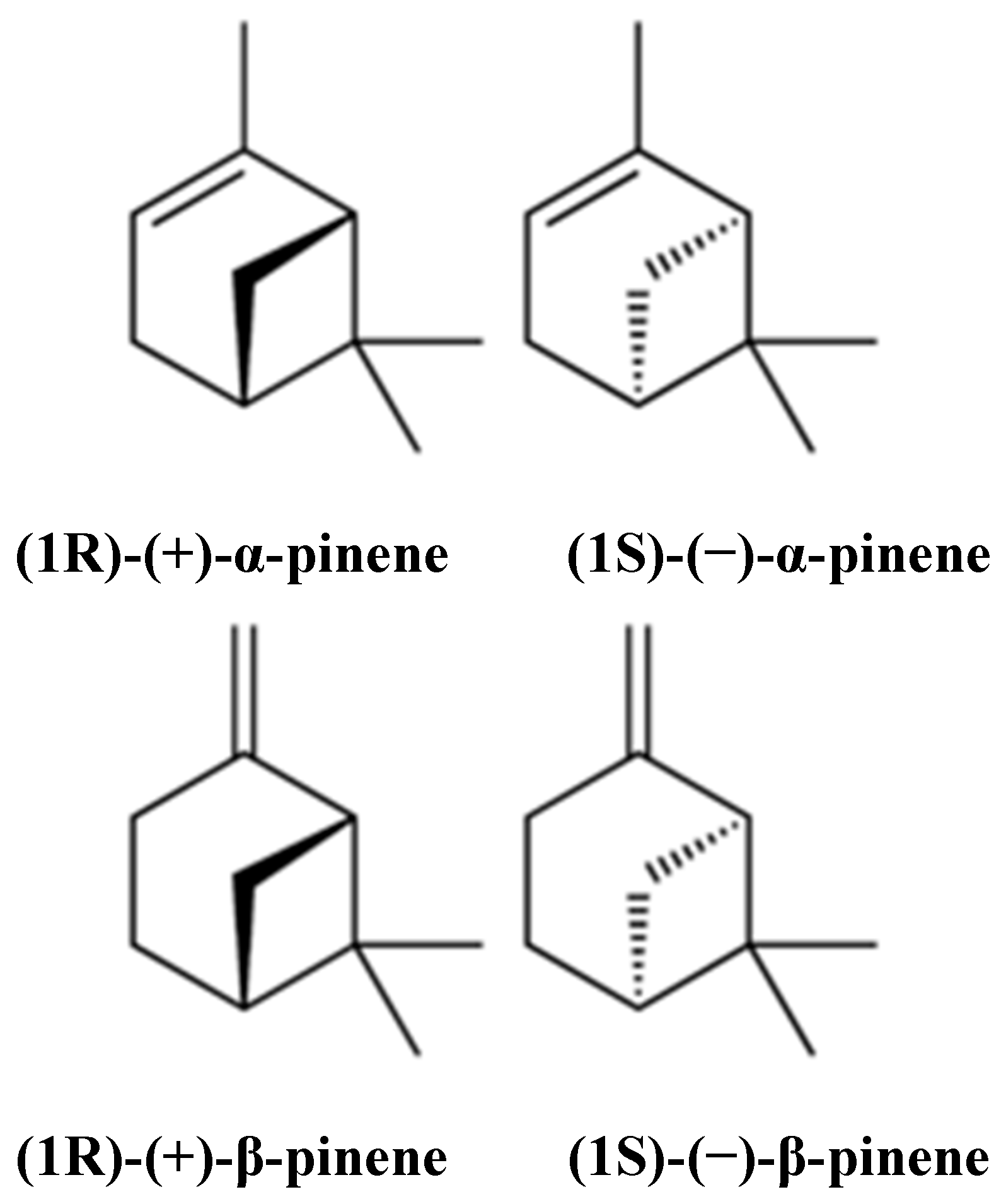
1. Introduction
2. Preclinical Pharmacological Activities of α- and β-Pinene
2.1. Antibiotic Resistance Modulation
2.2. Anticoagulative Activities
2.3. Antitumor Activity
2.4. Genomic Instability
2.5. Cytogenetic and Oxidative Effects
2.6. Gastroprotective Effect
2.7. Anxiolytic-Like Effects
2.8. Neuroprotective Activities
2.9. Cytoprotective Activity against H2O2-Stimulated Oxidative Stress
2.10. Inhibitory Effect on the Growth of Endocarditis Disease
2.11. Antimicrobial and Antimalarial Effects
2.12. Anti-Leishmania Activity
2.13. Effect on Cytochrome P-450 Levels
2.14. Effect on Pancreatitis
2.15. Porphyrogenic Properties
2.16. Protective Effect Against Cytotoxicity
2.17. Allergic Rhinitis
2.18. Anticonvulsant Effects
2.19. Anti-Inflammatory and Analgesic Properties
2.20. Effect on Stress Stimulated Hyperthermia
2.21. Permeability Glycoprotein (PgP) Transporter
2.22. Effect on Pulpal Pain (Dental Pain)
2.23. Sensory Irritation
2.24. Toxicokinetic Effect
2.25. Treatment of IgA Nephropathy
2.26. Insecticidal or Larvicidal Effects
3. Bioavailability of α-Pinene and β-Pinene
3.1. Dermal Application
3.2. Inhalation
3.3. Oral Administration
4. In Vivo Biological Activity
4.1. Anti-Cancer Activity
4.2. Anti-Allergic Activity
4.3. Anti-Inflammatory Activity
4.4. Antimicrobial Activity
5. α-Pinene and β-Pinene in Clinical Studies
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winnacker, M. Pinenes: Abundant and Renewable Building Blocks for a Variety of Sustainable Polymers. Angew. Chem. Int. Ed. 2018, 57, 14362–14371. [Google Scholar] [CrossRef]
- Vespermann, K.A.; Paulino, B.N.; Barcelos, M.C.; Pessoa, M.G.; Pastore, G.M.; Molina, G. Biotransformation of alpha- and beta-pinene into flavor compounds. Appl. Microbiol. Biotechnol. 2017, 101, 1805–1817. [Google Scholar] [CrossRef]
- Berger, R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin, Germany; New York, NY, USA, 2007; p. 648. [Google Scholar]
- Erman, M.B.; Kane, B.J. Chemistry around pinene and pinane: A facile synthesis of cyclobutanes and oxatricyclo-derivative of pinane from cis- and trans-pinanols. Chem. Biodivers. 2008, 5, 910–919. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.; Lopes, P.M.; de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of alpha-pinene and beta-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Sybilska, D.; Kowalczyk, J.; Asztemborska, M.; Ochocka, R.J.; Lamparczyk, H. Chromatographic studies of the enantiomeric composition of some therapeutic compositions applied in the treatment of liver and kidney diseases. J. Chromatogr. A 1994, 665, 67–73. [Google Scholar] [CrossRef]
- Alma, M.H.; Nitz, S.; Kollmannsberger, H.; Digrak, M.; Efe, F.T.; Yilmaz, N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish pistachio (Pistacia vera L.). J. Agric. Food Chem. 2004, 52, 3911–3914. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Tang, F.D.; Mao, G.G.; Bian, R.L. Effect of alpha-pinene on nuclear translocation of NF-kappa B in THP-1 cells. Acta Pharmacol. Sin. 2004, 25, 480–484. [Google Scholar]
- Winnacker, M.; Rieger, B. Recent progress in sustainable polymers obtained from cyclic terpenes: Synthesis, properties, and application potential. ChemSusChem 2015, 8, 2455–2471. [Google Scholar] [CrossRef]
- Kamigaito, M.; Satoh, K. Sustainable vinyl polymers via controlled polymerization of terpenes. In Sustainable Polymers from Biomass; Tang, C., Ryu, C.Y., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 55–90. [Google Scholar] [CrossRef]
- Thomsett, M.R.; Moore, J.C.; Buchard, A.; Stockman, R.A.; Howdle, S.M. New renewably-sourced polyesters from limonene-derived monomers. Green Chem. 2019, 21, 149–156. [Google Scholar] [CrossRef]
- Manfredi, K.P. Terpenes. Flavors, Fragrances, Pharmaca, Pheromones By Eberhard Breitmaier (University of Bonn). J. Nat. Prod. 2007, 70, 711. [Google Scholar] [CrossRef]
- Satoh, K.; Nakahara, A.; Mukunoki, K.; Sugiyama, H.; Saito, H.; Kamigaito, M. Sustainable cycloolefin polymer from pine tree oil for optoelectronics material: Living cationic polymerization of β-pinene and catalytic hydrogenation of high-molecular-weight hydrogenated poly(β-pinene). Polym. Chem. 2014, 5, 3222–3230. [Google Scholar] [CrossRef]
- Almirall, M.; Montana, J.; Escribano, E.; Obach, R.; Berrozpe, J.D. Effect of d-limonene, alpha-pinene and cineole on in vitro transdermal human skin penetration of chlorpromazine and haloperidol. Arzneim. Forsch. 1996, 46, 676–680. [Google Scholar]
- van der Werf, M.J.; de Bont, J.A.M.; Leak, D.J. Opportunities in microbial biotransformation of monoterpenes. In Biotechnology of Aroma Compounds; Berger, R.G., Babel, W., Blanch, H.W., Cooney, C.L., Enfors, S.O., Eriksson, K.E.L., Fiechter, A., Klibanov, A.M., Mattiasson, B., Primrose, S.B., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 147–177. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Khalifaev, P.D.; Sharopov, F.S.; Bakri, M.; Habasi, M.; Safomuddin, A.; Numonov, S.; Aisa, H.A.; Setzer, W.N. Chemical composition of the essential oil from the roots of Ferula kuhistanica growing wild in Tajikistan. Nat. Prod. Commun. 2017, 12, 1–4. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Satyal, P.; Wink, M. Composition of the essential oil of Ferula clematidifolia. Chem. Nat. Compd. 2016, 52, 518–519. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). The Bacterial Challenge: Time to React; European Center for Disease Prevention and Control EMA: Solna, Sweden, 2009; Available online: http://www.ecdc.europa.eu/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_React.pdf (accessed on 14 November 2019).
- Centers for Disease Control and Prevention (CDC). Antibiotic Resistance Threats in the United States; U.S. Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013; pp. 1–114. Available online: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 14 November 2019).
- Kovac, J.; Simunovic, K.; Wu, Z.; Klancnik, A.; Bucar, F.; Zhang, Q.; Mozina, S.S. Antibiotic resistance modulation and modes of action of (-)-alpha-pinene in Campylobacter jejuni. PLoS ONE 2015, 10, e0122871. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.T.; Bociek, S.M.; Harries, P.C.; Jeffcoat, R.; Sissons, D.J.; Trudgill, P.W. Bacterial metabolism of alpha-pinene: Pathway from alpha-pinene oxide to acyclic metabolites in Nocardia sp. strain P18.3. J. Bacteriol. 1987, 169, 4972–4979. [Google Scholar] [CrossRef]
- Nanjing University of Chinese Medicine. Dictionary of Chinese Herbal Medicines; Shanghai Science and Technology Press: Shanghai, China, 2006; p. 1207. [Google Scholar]
- Yang, N.Y.; Zhou, G.S.; Tang, Y.P.; Yan, H.; Guo, S.; Liu, P.; Duan, J.A.; Song, B.S.; He, Z.Q. Two new alpha-pinene derivatives from Angelica sinensis and their anticoagulative activities. Fitoterapia 2011, 82, 692–695. [Google Scholar] [CrossRef]
- Alberg, A.J.; Brock, M.V.; Samet, J.M. Epidemiology of lung cancer: Looking to the future. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3175–3185. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2014; American Cancer Society: Atlanta, GA, USA, 2014. [Google Scholar]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of alpha-pinene and beta-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2015, 65, 214–218. [Google Scholar] [CrossRef]
- Rotem, R.; Heyfets, A.; Fingrut, O.; Blickstein, D.; Shaklai, M.; Flescher, E. Jasmonates: Novel anticancer agents acting directly and selectively on human cancer cell mitochondria. Cancer Res. 2005, 65, 1984–1993. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine 2012, 19, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. The phytochemistry and chemotherapeutic potential of Tasmannia lanceolata (Tasmanian pepper): A review. Pharmacogn. Commun. 2013, 3, 13–25. [Google Scholar]
- Elanur, A.; Hasan, T.; Fatime, G. Antioxidative, anticancer and genotoxicproperties of α-pinene on N2a neuroblastoma cells. Biologia 2013, 68, 1004–1009. [Google Scholar]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Kusuhara, M.; Urakami, K.; Masuda, Y.; Zangiacomi, V.; Ishii, H.; Tai, S.; Maruyama, K.; Yamaguchi, K. Fragrant environment with alpha-pinene decreases tumor growth in mice. Biomed. Res. 2012, 33, 57–61. [Google Scholar] [CrossRef]
- Chen, W.Q.; Xu, B.; Mao, J.W.; Wei, F.X.; Li, M.; Liu, T.; Jin, X.B.; Zhang, L.R. Inhibitory effects of alpha-pinene on hepatoma carcinoma cell proliferation. Asian Pac. J. Cancer Prev. 2014, 15, 3293–3297. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef]
- Yang, J.B.; Li, M.; Xie, J.J.; Yang, M.D.; Lu, X.S.; Wang, F.; Chen, W.Q. Effects of alpha-pinene extracted from pine needle on expression of miR-221 and its potential target genes in human hepatocellular carcinoma cells. China J. Chin. Mater. Med. 2016, 41, 3996–3999. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. alpha-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. alpha-Pinene Inhibits Human Prostate Cancer Growth in a Mouse Xenograft Model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C.; Zhang, Q.; Shan, Y.; Gu, W.; Wang, S. Design, synthesis and biological evaluation of novel beta-pinene-based thiazole derivatives as potential anticancer agents via mitochondrial-mediated apoptosis pathway. Bioorganic Chem. 2019, 84, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, I.; Caradonna, F.; Barbata, G.; Saverini, M.; Mauro, M.; Sciandrello, G. Genomic instability induced by alpha-pinene in Chinese hamster cell line. Mutagenesis 2012, 27, 463–469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turkez, H.; Aydin, E. In vitro assessment of cytogenetic and oxidative effects of alpha-pinene. Toxicol. Ind. Health 2016, 32, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Yesilada, E. Traditional medicine and gastroprotective crude drugs. J. Ethnopharmacol. 2005, 100, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Falcao, H.S.; Mariath, I.R.; Diniz, M.F.; Batista, L.M.; Barbosa-Filho, J.M. Plants of the American continent with antiulcer activity. Phytomedicine 2008, 15, 132–146. [Google Scholar] [CrossRef]
- Juca, D.M.; da Silva, M.T.; Junior, R.C., Jr.; de Lima, F.J.; Okoba, W.; Lahlou, S.; de Oliveira, R.B.; dos Santos, A.A.; Magalhaes, P.J. The essential oil of Eucalyptus tereticornis and its constituents, alpha- and beta-pinene, show accelerative properties on rat gastrointestinal transit. Planta Med. 2011, 77, 57–59. [Google Scholar] [CrossRef]
- Pinheiro Mde, A.; Magalhaes, R.M.; Torres, D.M.; Cavalcante, R.C.; Mota, F.S.; Oliveira Coelho, E.M.; Moreira, H.P.; Lima, G.C.; Araujo, P.C.; Cardoso, J.H.; et al. Gastroprotective effect of alpha-pinene and its correlation with antiulcerogenic activity of essential oils obtained from Hyptis species. Pharmacogn. Mag. 2015, 11, 123–130. [Google Scholar] [CrossRef]
- Memariani, Z.; Sharifzadeh, M.; Bozorgi, M.; Hajimahmoodi, M.; Farzaei, M.H.; Gholami, M.; Siavoshi, F.; Saniee, P. Protective effect of essential oil of Pistacia atlantica Desf. on peptic ulcer: Role of alpha-pinene. J. Tradit. Chin. Med. 2017, 37, 57–63. [Google Scholar] [CrossRef]
- Yang, H.; Woo, J.; Pae, A.N.; Um, M.Y.; Cho, N.C.; Park, K.D.; Yoon, M.; Kim, J.; Lee, C.J.; Cho, S. alpha-Pinene, a Major Constituent of Pine Tree Oils, Enhances Non-Rapid Eye Movement Sleep in Mice through GABAA-benzodiazepine Receptors. Mol. Pharmacol. 2016, 90, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, H.; Okada, N.; Kubohara, M.; Satou, T.; Masuo, Y.; Koike, K. Expression of BDNF and TH mRNA in the brain following inhaled administration of alpha-pinene. Phytother. Res. 2015, 29, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. In vitro neuroprotective potential of the monoterpenes alpha-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Nat. C. 2016, 71, 191–199. [Google Scholar] [CrossRef]
- Porres-Martinez, M.; Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Major selected monoterpenes alpha-pinene and 1,8-cineole found in Salvia lavandulifolia (Spanish sage) essential oil as regulators of cellular redox balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species and the central nervoussystem. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.M.; Lima, E.O.; Souza, E.L.; Diniz, M.F.; Trajano, V.N.; Medeiros, I.A. Inhibitory effect of α- and β-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria. Braz. J. Pharm. Sci. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- van Zyl, R.L.; Seatlholo, S.T.; van Vuuren, S.F. The biological activities of 20 nature identical essential oil constituents. J. Essent Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Baik, J.S.; Kim, S.S.; Lee, J.A.; Oh, T.H.; Kim, J.Y.; Lee, N.H.; Hyun, C.G. Chemical composition and biological activities of essential oils extracted from Korean endemic citrus species. J. Microbiol. Biotechnol. 2008, 18, 74–79. [Google Scholar]
- Dhar, P.; Chan, P.; Cohen, D.T.; Khawam, F.; Gibbons, S.; Snyder-Leiby, T.; Dickstein, E.; Rai, P.K.; Watal, G. Synthesis, antimicrobial evaluation, and structure-activity relationship of alpha-pinene derivatives. J. Agric. Food Chem. 2014, 62, 3548–3552. [Google Scholar] [CrossRef]
- Liao, S.; Shang, S.; Shen, M.; Rao, X.; Si, H.; Song, J.; Song, Z. One-pot synthesis and antimicrobial evaluation of novel 3-cyanopyridine derivatives of (-)-beta-pinene. Bioorg. Med. Chem. Lett. 2016, 26, 1512–1515. [Google Scholar] [CrossRef]
- Rivera-Yanez, C.R.; Terrazas, L.I.; Jimenez-Estrada, M.; Campos, J.E.; Flores-Ortiz, C.M.; Hernandez, L.B.; Cruz-Sanchez, T.; Garrido-Farina, G.I.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Anti-Candida Activity of Bursera morelensis Ramirez Essential Oil and Two Compounds, alpha-Pinene and gamma-Terpinene-An In Vitro Study. Molecules 2017, 22, 2095. [Google Scholar] [CrossRef]
- de Macedo Andrade, A.C.; Rosalen, P.L.; Freires, I.A.; Scotti, L.; Scotti, M.T.; Aquino, S.G.; de Castro, R.D. Antifungal Activity, Mode of Action, Docking Prediction and Anti-biofilm Effects of (+)-beta-pinene Enantiomers against Candida spp. Curr. Top. Med. Chem. 2018, 18, 2481–2490. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Eduardo, L.; Farias, T.C.; Ferreira, S.B.; Ferreira, P.B.; Lima, Z.N.; Ferreira, S.B. Antibacterial Activity and Time-kill Kinetics of Positive Enantiomer of alpha-pinene Against Strains of Staphylococcus aureus and Escherichia coli. Curr. Top. Med. Chem. 2018, 18, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.A.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.; Carneiro, S.M.; Carvalho, F.A. Syzygium cumini (L.) Skeels essential oil and its major constituent alpha-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.A.; Shephard, E.A.; Pike, S.F.; Rabin, B.R.; Phillips, I.R. The effect of terpenoid compounds on cytochrome P-450 levels in rat liver. Biochem. Pharmacol. 1988, 37, 2223–2229. [Google Scholar] [CrossRef]
- de Montellano, P.R.O. Cytochrome P-450; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Bae, G.-S.; Park, K.-C.; Choi, S.B.; Jo, I.-J.; Choi, M.-O.; Hong, S.-H.; Song, K.; Song, H.-J.; Park, S.-J. Protective effects of alpha-pinene in mice with cerulein-induced acute pancreatitis. Life Sci. 2012, 91, 866–871. [Google Scholar] [CrossRef]
- Bonkovsky, H.L.; Cable, E.E.; Cable, J.W.; Donohue, S.E.; White, E.C.; Greene, Y.J.; Lambrecht, R.W.; Srivastava, K.K.; Arnold, W.N. Porphyrogenic properties of the terpenes camphor, pinene, and thujone (with a note on historic implications for absinthe and the illness of Vincent van Gogh). Biochem. Pharmacol. 1992, 43, 2359–2368. [Google Scholar] [CrossRef]
- Bouzenna, H.; Hfaiedh, N.; Giroux-Metges, M.A.; Elfeki, A.; Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. = Biomed. Pharmacother. 2017, 93, 961–968. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Madahavan, N.R.; Agilan, B.; Ganesan, M.; Prasad, N.R.; Rathinaraj, P. Alpha-pinene attenuates UVA-induced photoaging through inhibition of matrix metalloproteinases expression in mouse skin. Life Sci 2019, 217, 110–118. [Google Scholar] [CrossRef]
- Nam, S.-Y.; Chung, C.-K.; Seo, J.-H.; Rah, S.-Y.; Kim, H.-M.; Jeong, H.-J. The therapeutic efficacy of α-pinene in an experimental mouse model of allergic rhinitis. Int. Immunopharmacol. 2014, 23, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Zamyad, M.; Abbasnejad, M.; Esmaeili-Mahani, S.; Mostafavi, A.; Sheibani, V. The anticonvulsant effects of Ducrosia anethifolia (Boiss) essential oil are produced by its main component alpha-pinene in rats. Arq. De Neuro Psiquiatr. 2019, 77, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Felipe, C.F.B.; Albuquerque, A.M.S.; de Pontes, J.L.X.; de Melo, J.I.V.; Rodrigues, T.; de Sousa, A.M.P.; Monteiro, A.B.; Ribeiro, A.; Lopes, J.P.; de Menezes, I.R.A.; et al. Comparative study of alpha- and beta-pinene effect on PTZ-induced convulsions in mice. Fundam. Clin. Pharmacol. 2019, 33, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.; Lee, B.S.; Kim, S.H.; et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-kappaB Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-alpha-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Li, X.J.; Yang, Y.J.; Li, Y.S.; Zhang, W.K.; Tang, H.B. alpha-Pinene, linalool, and 1-octanol contribute to the topical anti-inflammatory and analgesic activities of frankincense by inhibiting COX-2. J. Ethnopharmacol. 2016, 179, 22–26. [Google Scholar] [CrossRef]
- Akutsu, H.; Kikusui, T.; Takeuchi, Y.; Mori, Y. Effects of alpha-pinene odor in different concentrations on stress-induced hyperthermia in rats. J. Vet. Med Sci. 2003, 65, 1023–1025. [Google Scholar] [CrossRef][Green Version]
- Green, A.K.; Haley, S.L.; Barnes, D.M.; Dearing, M.D.; Karasov, W.H. Is alpha-pinene a substrate for permeability-glycoprotein in wood rats? J. Chem. Ecol. 2006, 32, 1197–1211. [Google Scholar] [CrossRef]
- Rahbar, I.; Abbasnejad, M.; Haghani, J.; Raoof, M.; Kooshki, R.; Esmaeili-Mahani, S. The effect of central administration of alpha-pinene on capsaicin-induced dental pulp nociception. Int. Endod. J. 2019, 52, 307–317. [Google Scholar] [CrossRef]
- Kasanen, J.P.; Pasanen, A.L.; Pasanen, P.; Liesivuori, J.; Kosma, V.M.; Alarie, Y. Stereospecificity of the sensory irritation receptor for nonreactive chemicals illustrated by pinene enantiomers. Arch. Toxicol. 1998, 72, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, G.D.; Larsen, S.T.; Hougaard, K.S.; Hammer, M.; Wolkoff, P.; Clausen, P.A.; Wilkins, C.K.; Alarie, Y. Mechanisms of acute inhalation effects of (+) and (-)-alpha-pinene in BALB/c mice. Basic Clin. Pharmacol. Toxicol. 2005, 96, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.A.; Hagberg, M.T.; Löf, A.; Wigaeus-Hjelm, E.; Zhiping, W. Uptake, distribution and elimination of a-pinene in man after exposure by inhalation. Scand. J. Work Env. Health 1990, 16, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pan, J.; Sun, C.; Zhou, J.; Li, N.A. Clinical effects of perazine ferulate tablets combined with eucalyptol limonene pinene enteric soft capsules for treatment of children with IgA nephropathy. Exp. Ther. Med. 2016, 12, 169–172. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Hoti, S.L.; Bhattacharyya, A.; Benelli, G. Eugenol, alpha-pinene and beta-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016, 115, 807–815. [Google Scholar] [CrossRef]
- Haselton, A.T.; Acevedo, A.; Kuruvilla, J.; Werner, E.; Kiernan, J.; Dhar, P. Repellency of alpha-pinene against the house fly, Musca domestica. Phytochemistry 2015, 117, 469–475. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Wink, M.; Setzer, W.N. Radical scavenging and antioxidant activities of essential oil components—An experimental and computational investigation. Nat. Prod. Commun. 2015, 10, 153–156. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Electronic code of federal regulations. In Title 21 Food and Drugs; U.S. Food and Drug Administration: Washington, DC, USA, 2018. [Google Scholar]
- Köse, E.O.; Deniz, I.G.; Sarıkürkçü, C.; Aktaş, Ö.; Yavuz, M. Chemical composition, antimicrobial and antioxidant activities of the essential oils of Sideritis erythrantha Boiss. and Heldr.(var. erythrantha and var. cedretorum PH Davis) endemic in Turkey. Food Chem. Toxicol. 2010, 48, 2960–2965. [Google Scholar]
- Schmidt, L.; Belov, V.N.; Göen, T. Sensitive monitoring of monoterpene metabolites in human urine using two-step derivatisation and positive chemical ionisation-tandem mass spectrometry. Anal. Chim. Acta 2013, 793, 26–36. [Google Scholar] [CrossRef]
- Schmidt, L.; Göen, T. Human metabolism of α-pinene and metabolite kinetics after oral administration. Arch. Toxicol. 2017, 91, 677–687. [Google Scholar] [CrossRef]
- Southwell, I.; Flynn, T.; Degabriele, R. Metabolism of α-and β-pinene, β-cymene and 1, 8-cineole in the brushtail possum, Trichosurus vulpecula. Xenobiotica 1980, 10, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Kohlert, C.; Van Rensen, I.; März, R.; Schindler, G.; Graefe, E.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Cal, K.; Sopala, M. Ex vivo skin absorption of terpenes from Vicks VapoRub ointment. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2008, 14, Pi19–Pi23. [Google Scholar]
- Schmitt, S.; Schaefer, U.; Sporer, F.; Reichling, J. Comparative study on the in vitro human skin permeation of monoterpenes and phenylpropanoids applied in rose oil and in form of neat single compounds. Die Pharm. 2010, 65, 102–105. [Google Scholar]
- Falk, A.; Gullstrand, E.; Löf, A.; Wigaeus-Hjelm, E. Liquid/air partition coefficients of four terpenes. Occup. Environ. Med. 1990, 47, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Filipsson, A.F. Short term inhalation exposure to turpentine: Toxicokinetics and acute effects in men. Occup. Environ. Med. 1996, 53, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.-O.; Eriksson, K.; Falk, A.; Löf, A. Renal elimination of verbenols in man following experimental α-pinene inhalation exposure. Int. Arch. Occup. Environ. Health 1992, 63, 571–573. [Google Scholar] [CrossRef]
- Bourgou, S.; Pichette, A.; Marzouk, B.; Legault, J. Bioactivities of black cumin essential oil and its main terpenes from Tunisia. S. Afr. J. Bot. 2010, 76, 210–216. [Google Scholar] [CrossRef]
- Lampronti, I.; Saab, A.M.; Gambari, R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int. J. Oncol. 2006, 29, 989–995. [Google Scholar] [CrossRef]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1). HNO 2011, 59, 1203–1208. [Google Scholar] [CrossRef]
- Sadlon, A.E.; Lamson, D.W. Immune-modifying and antimicrobial effects of Eucalyptus oil and simple inhalation devices. Altern. Med. Rev. 2010, 15, 33–43. [Google Scholar] [PubMed]
- Wei, Q.; Harada, K.; Ohmori, S.; Minamoto, K.; Wei, C.; Ueda, A. Toxicity study of the volatile constituents of Myoga utilizing acute dermal irritation assays and the Guinea-pig Maximization test. J. Occup. Health 2006, 48, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S.; Ip, S.P. Pancreatic acinar cell: Its role in acute pancreatitis. Int. J. Biochem. Cell Biol. 2006, 38, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Kapsaski-Kanelli, V.; Evergetis, E.; Michaelakis, A.; Papachristos, D.; Myrtsi, E.; Koulocheri, S.; Haroutounian, S. “Gold” Pressed Essential Oil: An Essay on the Volatile Fragment from Citrus Juice Industry By-Products Chemistry and Bioactivity. Biomed Res. Int. 2017. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakis, A. Evaluation of bioefficacy of three Citrus essential oils against the dengue vector Aedes albopictus (Diptera: Culicidae) in correlation to their components enantiomeric distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef]
- Vourlioti-Arapi, F.; Michaelakis, A.; Evergetis, E.; Koliopoulos, G.; Haroutounian, S.A. Essential oils of indigenous in Greece six Juniperus taxa: Chemical composition and larvicidal activity against the West Nile virus vector Culex pipiens. Parasitol. Res. 2012, 110, 1829–1839. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.; Viljoen, A. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Chen, I.-N.; Chang, C.-C.; Ng, C.-C.; Wang, C.-Y.; Shyu, Y.-T.; Chang, T.-L. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant. Foods Hum. Nutr. 2008, 63, 15–20. [Google Scholar] [CrossRef]
- Belman, S.; Solomon, J.; Segal, A.; Block, E.; Barany, G. Inhibition of soybean lipoxygenase and mouse skin tumor promotion by onion and garlic components. J. Biochem. Toxicol. 1989, 4, 151–160. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A. Pharmacological interactions of essential oil constituents on the viability of microorganisms. Nat. Prod. Commun. 2010, 5, 1381–1386. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal properties of terpenes found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Dorow, P.; Weiss, T.; Felix, R.; Schmutzler, H. Effect of a secretolytic and a combination of pinene, limonene and cineole on mucociliary clearance in patients with chronic obstructive pulmonary disease. Arzneim. Forsch. 1987, 37, 1378–1381. [Google Scholar]
- Matthys, H.; de Mey, C.; Carls, C.; Ryś, A.; Geib, A.; Wittig, T. Efficacy and tolerability of myrtol standardized in acute bronchitis. A multi-centre, randomised, double-blind, placebo-controlled parallel group clinical trial vs. cefuroxime and ambroxol. Arzneim. Forsch. 2000, 50, 700–711. [Google Scholar]
- Rossini, C.; Castillo, L.; Gonzalez, A. Plant extracts and their components as potential control agents against human head lice. Phytochem. Rev. 2008, 7, 51–63. [Google Scholar] [CrossRef]
- Ghavami, M.B.; Ahmadi, S. Effectiveness of eucalyptus and cinnamon essential oils compared to permethrin in treatment of head lice infestation. J. Zanjan Univ. Med. Sci. Health Serv. 2017, 25, 86–98. [Google Scholar]
- Benny, A.; Thomas, J. Essential oils as treatment strategy for Alzheimerʼs disease: Current and future perspectives. Planta Med. 2019, 85, 239–248. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Bollen, C.; Perry, E.K.; Ballard, C. Salvia for dementia therapy: Review of pharmacological activity and pilot tolerability clinical trial. Pharmacol. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Tildesley, N.T.; Kennedy, D.O.; Perry, E.K.; Ballard, C.G.; Savelev, S.; Wesnes, K.A.; Scholey, A.B. Salvia lavandulaefolia (Spanish sage) enhances memory in healthy young volunteers. Pharm. Biochem. Behav. 2003, 75, 669–674. [Google Scholar] [CrossRef]
- Porres-Martinez, M.; Accame, E.C.A.; Gomez-Serranillos, M.P. Pharmacological activity of Salvia lavandulifolia and chemical components of its essential oil. A review. Lazaroa 2013, 34, 237–254. [Google Scholar] [CrossRef]
- Perry, N.; Court, G.; Bidet, N.; Court, J.; Perry, E. European herbs with cholinergic activities: Potential in dementia therapy. Int J. Geriatr. Psychiatry 1996, 11, 1063–1069. [Google Scholar] [CrossRef]
- Perry, N.; Houghton, P.J.; Jenner, P. Inhibition of erythrocyte acetylcholinesterase by droplet counter-current chromatography fractions of Salvia lavandulaefolia oil. J. Pharm. Pharmacol. 1997, 49, 34. [Google Scholar]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Biochem Pharm. Behav 2003, 75, 661–668. [Google Scholar] [CrossRef]
- Lopresti, A.L. Salvia (Sage): A review of its potential cognitive-enhancing and protective effects. Drugs R D 2017, 17, 53–64. [Google Scholar] [CrossRef]
- Moss, L.; Rouse, M.; Wesnes, K.A.; Moss, M. Differential effects of the aromas of Salvia species on memory and mood. Hum. Psycho Pharm. Clin. Exp. 2010, 25, 388–396. [Google Scholar] [CrossRef]
- Máthé, I.; Hohmann, J.; Janicsák, G.; Nagy, G.; Dora, R. Chemical diversity of the biological active ingredients of Salvia officinalis and some closely related species. Acta Pharm. Hung. 2007, 77, 37–45. [Google Scholar]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-bdour, T.H.; Salgueiro, L. Essential oil of common sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940. [Google Scholar] [CrossRef]
- Tosun, A.; Khan, S.; Kim, Y.S.; Calín-Saánchez, A.; Hysenaj, X.; Carbonell-Barrachina, A.A. Essential oil composition and anti-inflammatory activity of Salvia officinalis L. (Lamiaceae) in murin macrophages. Trop. J. Pharm. Res. 2014, 13, 937–942. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Dodd, F.L.; Robertson, B.C.; Okello, E.J.; Reay, J.L.; Scholey, A.B. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J. Psychopharmacol. 2011, 25, 1088–1100. [Google Scholar] [CrossRef]
| S. No. | Source/Species | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|
| α-pinene | ||||||
| 1 | Sigma Aldrich | Campylobacter jejuni | - | Broth microdilution and ethidium bromide deposition | Modulation of antibiotic resistance, by reducing MIC value of ciprofloxacin, erythromycin, and triclosan, up to 512 times. α-pinene also affected antimicrobial efflux systems | [21] |
| 2 | - | Nocardia sp. Strain (P18.3), Pseudomonas putida PX1 (NCIB 10684), Pseudomonas sp. strain PIN18 (NCIB 10687), and P. fluorescens NCIB 11671 | - | Strains were cultured into agar slants with α-pinene (3 g/L in media), and strains growth was recorded | Nocardia sp. growth (P18.3) was not remarkable; Pseudomonas strains (NCIB 10684, 10687, and 11,671 and PL) increased promptly when α-pinene (0.3%, v/v) was added | [22] |
| 24 | Citrus species | Propionibacterium acnes, Staphylococcus epidermidis | Peel EO | EO was isolated by hydrodistillation | EO demonstrated outstanding antibacterial properties against P. acnes and S. epidermidis | [55] |
| 26 | Sigma-Aldrich | Escherichia coli, Micrococcus luteus, Staphylococcus aureus, and Candida albicans | - | Bioautographic method MIC was measured | (+)-α-pinene exhibited modest activity. (−)-α-pinene was unable to display any activity. α-pinene and β-lactams revealed the highest effects. Although (−)-α-pinene revealed no positive activity, the derivatives like β-lactam, amino ester, and amino alcohol exhibited antimicrobial effects | [56] |
| 28 | Bursera morelensis | Candida albicans strains (ATCC 14065, ATCC 32354, donated strain, and CDBB-L-1003) | Stems (EO) | EO was extracted by hydrodistillation, and GC-MS was used to isolate compounds Disc diffusion and survival curve assay were used | Maximum antifungal activity was attributed to the EO and its constituent, namely, α-pinene. Minimum fungicidal concentration of EO was found to be 2 mg/mL. A slight reduction in C. albicans population was recorded after 12 h | [58] |
| 30 | - | Staphylococcus aureus and Escherichia coli | - | Disc diffusion test, broth microdilution, and bacterial death kinetics | Inhibition halos of 11 and 12 mm for gram-positive and -negative strains were obtained at 160 µL/mL, respectively. At 1.25 and 2.5 µL/mL, (+)-α-pinene was able to eliminate bacterial colonies formation at one time of exposure of 2 h for E. coli strain | [60] |
| 31 | Syzygium cumini | Swiss mice | Leaves (EO) | MTT assay Cytotoxic effect on macrophages was determined; cells were exposed to α-pinene and tested against Leishmania | Cytotoxic effect of α-pinene against promastigotes of Leishmania amazonensis was observed with different cell death percentages (93.7, 83.2, and 58.4%) at different concentrations (100, 50, and 25 mg/mL respectively) | [61] |
| 40 | - | House fly (Musca domestica) | - | Y-tube and house flies were selected for this test | Solution with lowest concentration did not show significant differences in Y-tube arm choice. (1S)-(-)-α-pinene had maximum repellent efficiency for house flies when compared to (1R)-(+)-α-pinene | [84] |
| 45 | Plectranthus barbatus | Malaria (Anophel es subpictus), dengue (Aedes albopictus), and Japanese encephalitis (Culex tritaeniorhynchus) mosquito vectors | EO (leaves) | GC and GC--MS were performed; larvicidal activity of EO (40, 80, 120, 160, and 200 µg/mL) and its constituents eugenol, α-pinene, and β-caryophyllene (12–100 µg/mL each) were determined by WHO methods. Mortality of larvae was measured at 24 h after exposure | EO showed substantial larvicidal effects with LC50 values of 84.20, 87.25, and 94.34 µg/mL for the selected mosquito species. For Anapheles subpictus, eugenol, α-pinene, and β-caryophyllene revealed larvicidal effects (LC50 = 25.45, 32.09, and 41.66 μg/mL), followed by Aedes albopictus (LC50 = 28.14, 34.09, and 44.77 μg/mL) and Culex tritaenior hynchus (LC50 = 30.80, 36.75, and 48.17 μg/mL, respectively) | [83] |
| β-pinene derivatives | ||||||
| 27 | - | Klebsiella pneumoniae, Enterobacter aerogenes, S. aureus, S. epidermidis, and Candida albicans | - | 25 3-cyanopyridine compounds of β-pinene were prepared; MIC value was recorded using serial two-fold dilution method | MICs values of all derivatives ranged from 15.6 to 125 mg/l | [57] |
| 29 | - | Candida spp. | - | MIC and MFC values and microbial death curve after treatment with (+)-β-pinene enantiomers | MIC values ranged from <56.25–1800 µmol/L (+)-β-pinene. After ergosterol addition, MIC value of (+)-β-pinene was not altered, but was altered with sorbitol addition. (+)-β-pinene displayed anti-biofilm activity against multiple Candida species | [59] |
| α- and β-pinene | ||||||
| 22 | Dep. Pharmaceutical Sciences, Ponta Grossa, Brazil | Gram-positive bacteria (Staphylococcus aureus, S. epidermidis, S. pneumoniae, and S. pyogenes) | - | MIC value, viable cells count | All studied bacterial strains were sensitive to α- and β-pinene. MIC values ranged from 5 (α-pinene x S. epidermidis SSI 1; ATCC 12228; S. pyogenes ATCC 19,615; and S. pneumoniae) to 40 μL/mL (β-pinene x S. epidermidis ATCC 12228). Few bacterial strains were resistant antibiotics, mainly gentamicin. S. aureus was resistant to α- and β-pinene | [53] |
| 23 | Sigma-Aldrich | Antimicrobial: Escherichia coli (ATCC 11775, Staphylococcus aureus (ATCC 25923), Bacillus cereus (ATCC 11778), and Candida albicans (ATCC 10231). Antimalarial: Plasmodium falciparum (FCR-3) | - | Disc diffusion method. MIC was investigated. Antimalarial properties were analyzed using the tritiated hypoxanthine incorporation assay | (+)-β-pinene was approximately two to 12 times more effective as compared to (+)-α-pinene against both gram-positive and negative bacteria, as well as C. albicans. (+)-α-pinene shows 250-fold more antimalarial activity than (+)-β-pinene | [54] |
| 25 | Sigma-Aldrich | Candida albicans, Cryptococcus neoformans, Rhizopus oryzae, and methicillin-resistant Staphylococcus aureus (MRSA) | - | Two-fold serial dilution method was used to evaluate MIC for all the strains | MIC values of α- and β-pinene enantiomers were found to be from 117 to 6250 µg/mL. C. albicans exhibited higher sensitivity to α- and β-pinene enantiomers than MRSA. Positive enantiomers possess capability to kill 100% of C. albicans in 60 min., and 6 h was required for total killing of MRSA | [5] |
| S. No. | Compound | Source/Species | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|---|
| Anticoagulative/Antiplatelet | |||||||
| 3 | α-pinene derivatives (6β,9-dihydroxy-(+)-α-pinene and 9-hydroxy-(+)-α-pinene-6β-O-D-glucoside) | Angelica sinensis (Oliv.) Diels | New Zealand white rabbits | Aerial parts | Two α-pinene derivatives were extracted from aerial parts (10 g). Thrombin time and platelet aggregation methods were used to establish the anticoagulative properties in vitro | Isolated α-pinene derivatives slightly prolonged thrombin time and strongly prevented platelet aggregation. This effect seems to be due to prevention of thromboxane A2 synthesis or agitation of Ca2+ in platelet | [24] |
| Anti-Inflammatory | |||||||
| 41 | α-pinene enantiomers | Juniperus oxycedrus | Human chondrocyte | EO | Chondrocytes were cultured and exposed to noncytotoxic doses of α-pinene enantiomers | (+)-α-pinene (1) shows maximum suppression of IL-1β-induced inflammatory and catabolic pathways | [74] |
| 42 | α-pinene | - | Male C57BL/6 mice (peritoneal macrophages) | - | Cytotoxicity was determined by MTT method. Cytokine assays were executed for IL-6 and TNF-α by following modified ELISA method. Western blotting was used to analyze protein expression | Up to 20 µL, α-pinene was not cytotoxic. α-pinene reduced nitrite oxide, and IL-6 and TNF-α formation, in macrophages of rats. MAPK/NF-kB pathway activation plays an essential role in inflammatory activities. α-pinene exhibited inhibitory activity on NF-kB activation | [73] |
| 43 | α-pinene | Frankincense oil (Boswellia carterii) | Kunming mice | - | Frankincense oil was extracted from Boswellia carterii, and three compounds, namely, α-pinene, linalool, and 1-octanol, were isolated using GC-MS. Frankincense oil, water extracts, and their constituents were screened against xylene-stimulated edema and formalin-sensitized hind paw edema in rat model for determining the anti-inflammatory and anti-analgesic properties. | Frankincense oil possesses higher anti-inflammatory and anti-analgesic effects than rats administered with water extract. Mixtures of the three constituents have higher pharmacological properties on hind-paw inflammation and COX-2 over expression than used individually | [75] |
| 46 | α-pinene | Sigma-Aldrich | Wood rats (Neotoma species) | Selected wood rats were sacrificed and intestine removed rapidly from stomach | α-pinene is not a PgP substrate | [77] | |
| 47 | α-pinene | Wistar rats | - | Selected rats were cannulated via their lateral ventricles for capsaicin administration (100 µg). α-pinene at various concentrations (0.1, 0.2, and 0.4 µM) was administered | 0.2 and 0.4 μM concentrations of α-pinene were able to decrease nociception. A marked increase in COX-2 expression in capsaicin-treated rats was observed, which was prohibited by 0.4 μM α-pinene | [78] | |
| S. No. | Source/Species | Model | Plant Portion | Method | Result | Ref. |
|---|---|---|---|---|---|---|
| α-pinene | ||||||
| 4 | Schinus terebinthifolius | Male C57BL/6 mice and B16F10 murine melanoma cell line | Fruits | α-pinene was extracted from ripped fruits and injected into infected mice. Selected cells were cultured and maintained in culture medium | α-pinene-stimulated apoptosis was by early disruption of mitochondrial potential, ROS formation, enhanced caspase-3 activity, heterochromatin deposition, DNA fragmentation, and phosphatidylserine exposure on cell surface | [33] |
| 5 | - | C57/BL6 mice | - | α-pinene under aesthetic chamber and mouse cage, and in vitro effects | No effect was found on melanoma cell proliferation in mice under in vitro use of α-pinene | [34] |
| 6 | Pinus massoniana | Hepatoma carcinoma BEL-7402 cells | Needles | Selected cells were cultured and maintained in RPMI-1640 medium. Cell viability was checked by MTT assay. Cell cycle arrest was observed by flow cytometry. Western blotting was performed to know protein expression | α-pinene prevented BEL-7402 cells by arresting cell growth at G2/M, down regulating Cdc25C mRNA and protein expression, and decreasing cycle dependence on kinase 1(CDK1) action | [35] |
| 7 | Pinus massoniana | Hepatoma carcinoma BEL-7402 cells | - | α-pinene was isolated from pine needles. Selected cells were cultured and maintained in RPMI-1640 medium. MTT and flow cytometry assays were used for determination of cytotoxicity and cell cycle regulation, respectively. | Liver cancer cell growth was prevented in vitro and in vivo (respectively, 79% and 69.1% inhibitory rate); Chk1 and Chk2 levels were up-regulated; and Cyclin B, CDC25 and CDK1 levels were down-regulated | [36] |
| 9 | Pine | Human hepatocellular carcinoma cells (HepG2 cell) | Pine needle | HepG2 cell was administered with α-pinene and cell cycle alteration was analyzed by flow cytometry | α-pinene prevented HepG2 cells proliferation dose-dependently. α-pinene arrested HepG2 cells at G2/M phase. miR-221 expression was down-regulated in HepG2 cell treated with α-pinene | [37] |
| 10 | - | HepG2, MCF-7, A549, and PC-12 cancer cell lines | - | Cell viability was determined by MTT assay, apoptosis and cell cycle analyses were conducted using flow cytometry | α-pinene inhibited miR221 expression, leading to G2/M-phase cell cycle arrest and activation of CDKN1B/p27-CDK1 and ATM-p53-Chk2 pathways that suppress human hepatoma tumor progression | [38] |
| 11 | - | Mouse xenograft model | Cytotoxicity was analyzed using MTT assay, and apoptosis and cell cycle study were performed in vitro by flow cytometry | α-Pinene prevented human prostate cancer cell growth and stimulated apoptosis and cell cycle arrest in the cell line-based model. α-Pinene administration stimulated apoptosis in xenograft tumors as measured by TUNEL | [39] | |
| 13 | Sigma-Aldrich | Chinese hamster (V79-Cl3) cell line | - | Cells (3 × 105 per dish) were exposed at varying doses of α-pinene (0, 25, 30, 35, 40, and 50 µM) for 1 h | Cells morphological analysis revealed a significant enhancement in cell. Apoptotic cells were found at 40 and 50 µM. Genetic instability was stimulated by α-pinene, interfering in mitotic process and causing irregularity in 50% of cells. α-pinene stimulated oxidative stress and led to DNA damage | [41] |
| β-pinene-based thiazole derivative | ||||||
| 12 | - | Human cervical carcinoma HeLa cells, colon cancer CT-26, and human hepatocarcinoma SMMC-7721 cell lines | - | Mechanism of compound 5 g (β-pinene-based thiazole derivatives) on cytotoxicity, DAPI, Annexin-V/PI, JC-1, DCFDA staining, and Western blot assay were performed | Studied compound prevented HeLa cells proliferation through apoptosis stimulation and cell cycle arrest at G0/G1 phase, dose-dependently. Studied compound increased ROS level; caused a reduction in mitochondrial membrane potential; enhanced mitochondrial cytochrome C discharge; and impacted Bax, Bcl-2, caspase-3, and caspase-9 expression | [40] |
| α- and β-pinene | ||||||
| 8 | - | A-549 and H 460 cancer cell line | - | Selected cells were maintained in RMPI-1640 medium. MTT assay was used to analize cell viability. Cell cycle regulation was checked by flow cytometry | A significant inhibitory effect of the mixture of paclitaxel (PAC) with α-pinene or β-pinene was recorded on non-small-cell lung cancer cell lines | [27] |
| S. No. | Source/Species | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|
| α-pinene | ||||||
| 14 | Sigma Aldrich | Cultured human blood cells | - | Varying doses of α-pinene (at 0, 10, 25, 50, 75, 100, 150, and 200 mg/L doses) were administered in human blood cells for 24 and 48 h. Cytotoxicity was evaluated using LDH and MTT methods. DNA damage was detected using micronucleus assay, chromosomal aberration, and 8-oxo-2-deoxyguanosine (8-OH-dG). Total antioxidant capacity (TAC) and total oxidative stress (TOS) were measured. | Reduced cell viability was recorded by α-pinene (200 mg/L) administration. No changes were detected in the rates of genotoxicity endpoints. Dose-dependent changes were recorded in TAC and TOS levels. TAC levels were enhanced after supplementation with α-pinene (25 and 50 mg/L), while TOS level were reduced only at 200 mg/L of α-pinene on human lymphocytes | [42] |
| 32 | Aldrich chemicals | Sprague–Dawley rats | - | Pinene dissolved in 10% ethanol and 90% corn oil at 40 mg/kg b.w. was injected three times into healthy mice with 180–200 g weight. Comparative assessments of these mice were performed with few other mice administered with phenobarbital (0.9% NaCl). Control mice received a vehicle (10% ethanol and 90% corn oil) | No visible alterations were recorded in liver microsomal membrane proteins of mice after administration of the different terpenoids. No effect was found in the amount of cytochrome present in mice liver. Terpenoids administered mice had remarkable stimulation on PB P-450 | [62] |
| 34 | Sigma chemicals | Rat small intestine epithelial (IEC-6) cells | - | DPPH assay was examined at varying doses of α-pinene (25, 50, 100, 200, 300, and 400 µg/mL). IEC-6 cells were exposed in 10 mM aspirin (A) with and without α-pinene for 24 h. SOD, mitochondrial SOD, and glutathione activities were assessed | With enhancing doses of α-pinene until a maximum dose (400 µg/mL) was reached, the anti-DPPH activity was found to increase. FRAP activity was enhanced by increasing the dose of α-pinene (up to 300 µg/mL). Lower dose of α-pinene was unable to display any effect on cell viability. Exposure of aspirin with α-pinene displayed an expansion in cytotoxicity, compared to exposure of aspirin alone. Aspirin caused a negative alteration in cell morphology; however, exposure to aspirin with α-pinene did not lead to morphological changes | [66] |
| 35 | - | Human skin epidermal keratinocytes (HaCat cells) | - | HaCat cells were kept in DMEM administration and then divided into four groups, i.e., non-irradiated control cells, α-pinene (30 µm)-treated cells, UVA (10 J/cm2)-irradiated cells, and α-pinene-pretreated (30 min before) and UVA-irradiated cells. Cellular damage was caused by the stimulation of UVA-irradiation (10 J/cm2) | Up to 30 µm α-pinene, no cell death was observed. Cell viability decreased significantly after UVA exposure. UVA-stimulated cytotoxicity was inhibited by α-pinene pretreatment. UVA irradiation enhanced ROS formation. However, α-pinene pretreatment significantly inhibited ROS formation. UVA-exposed cells exhibited higher peroxidation levels, decreased by α-pinene | [67] |
| 36 | Sigma chemicals | Swiss Albino mice | - | Cell damages was triggered by UVA-irradiation (10 J/cm2 per day) for 10 days. Before-exposure rats were administered with α-pinene (100 mg kg/b.wt). Antioxidant enzymes and oxidative stress were analyzed. In the rat skin, histopathological analysis was also carried out | UVA exposure decreased the level of SOD, CAT, GPx, and GSH in mouse skin, and increased ROS formation. Peroxidation level was higher in UVA-exposed rat, compared to non-irradiated control and α-pinene-alone-administered mice. α-pinene administration before UVA-exposure significantly enhanced SOD, CAT, GPx, and GSH activities, and significantly decreased the level of lipid peroxidation. α-pinene-treated mice exhibited greater iNOS and VEGF expression than non-treated control rats | [68] |
| β-pinene | ||||||
| 52 | Sigma-Aldrich | In vitro | DPPH, ABTS, and FRAP assays | IC50 values for DPPH and ABTS were 3116.3 μg/mL and 2245.0 μg/mL, respectively. FRAP value was 6.5 μM Fe/mg pinene. | [85] | |
| S. No. | Source/Species | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|
| α-pinene | ||||||
| 16 | Sigma-Aldrich and Hyptis species | Swiss mice | EO | Different doses of ethanol and indomethacin (10, 30, and 100 mg/kg) were used to induced gastric ulcers. Acute gastric lesions were introduced into rats, and these rats fasted for 12 h. After that, rats were administered with 0.5 mL of vehicle (0.1% tween-80), ranitidine (40 mg/kg), and α-pinene (10, 30, and 100 mg/kg) dissolved in vehicle | α-pinene decreased ethanol-induced gastric mucosa lesion and produced gastroprotective effects similar to ranitidine (40 mg/kg). There were no remarkable variations between lesions area of α-pinene and vehicle-pretreated mice | [46] |
| 17 | Pistacia atlantica | Wistar strain mal albino rats | Oleoresin (EO) | EO was supplemented with varying doses in the selected mice. Mice were kept under observation after 72 h to determine toxicity (restlessness, dullness, and agitation). 80% ethanol was supplemented. Rats were sacrificed 2 h after to remove stomachs. Gastric ulcers were determined using microscopy. H. pylori strains were cultured | EO was harmless up to 2000 mg/kg. Strains of H. pylori were sensitive to EO. MIC values ranged from 0.275 to 1.100 mg/mL. EO considerably decreased ethanol-stimulated peptic ulcer. Pretreatment with EO reduced ethanol-stimulated gastric tissue damage and necrosis | [47] |
| 19 | Sigma-Aldrich | C57BL/6 mice | - | Mice were fasted for 18 h, followed by stimulation of acute pancreatitis (AP). AP was treated in every h (for 6 h) by cerulein (50 µg/kg i.p.). α-pinene was vaccinated at varying doses before the first cerulein injection. | After α-pinene stimulation, PW/BW proportion was reduced. Lipase and amylase levels were enhanced in serum during cerulein-induced AP, whereas α-pinene decreased them | [64] |
| 33 | Aldrich cehmicals | Barred Rock Chickens | - | Livers of 16- to 18-day-old embryos of identified chickens were cultured and compared with white Leghorn embryos for knowing the behavior. Porphyrins were analyzed fluorimetrically | α-pinene formed some amount of porphyrins in chick embryo liver cells. α-pinene led to the deposition of 100-150 porphyrins/mg (copro- and protoporphyrins) protein at the highest screened dose (1 mM). | [65] |
| α- and β-pinene | ||||||
| 15 | Eucalyptus tereticornis | Male Wistar rats | EO of whole plant | Liquid test meal comprising phenol red was supplemented, and gastric emptying was analyzed after varying time intervals | Studied species and their components reduced gastric retention in mice, and α- and β-pinene enhanced gastric tonus in anesthetized rats | [45] |
| S. No. | Source/Species | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|
| α-pinene | ||||||
| 18 | Santa Cruz Biotechnology Inc. (Dallas, TX, USA) | Mice | - | α-pinene and zolpidem were supplemented orally pre-pentobarbital injection (45 mg/kg) | α-pinene displayed sleep improving activity through a direct binding to GABAA-benzodiazepine receptors (GABAA-BZD). α-pinene (12.5, 25, 50, and 100 mg/kg) reduced sleep latency and enhanced the duration of NREMS without any action on REMS and delta effects | [48] |
| 19 | Tokyo Chemical Industry | Mice | - | Rats were exposed to α-pinene and water as negative control for 60/90 min. Followed by inhalation, quantitative measurement of α-pinene in brain and gene expression was undertaken. EPM test was performed for determining the anxiolytic-like effect in rats | Distance was enhanced (p < 0.001, d = 3.4, and 1-β = 0.98) when mice inhaled α-pinene for 60 min. α-pinene dose for 60 min in brain was higher when compared to 90 min. BDNF mRNA expression in olfactory bulb and hippocampus was almost similar at 60 min inhalation than at 90 min. TH mRNA expression in middle brain at 60 min was higher | [49] |
| 20 | Sigma-Aldrich | Rat pheochromocytoma cells (PC12) | - | PC12 viability was checked using MTT method. Cells were incubated for 30 min with DCFH-DA. Intracellular ROS formation was measured by DCFH-DA assay | α-pinene pretreatment led to cell viability loss and alteration in cell morphology. α-pinene prevented intracellular ROS production, and increased CAT, SOD, GPx, GR, and HO-1 expression | [50] |
| 21 | Salvia lavandulifolia | Human astrocytoma 373-MG cell line | Aerial parts | Cytotoxicity was evaluated using MTT method. DCFH-DA method was used to evaluate intracellular ROS formation. TBARS method was used for lipid peroxidation, and spectrometric techniques and Western blot for enzymatic activity and protein expression | Viability of α-pinene-treated cells (10–250 mM) was not reduced. Earlierα-pinene (at 10, 25, 50, and 100 mM dosed) administration enhanced cell viability in U373-MG dose-dependently. (IC50 = 79.70 mM). α-pinene pre-treatment preserved U373-MG cells against H2O2-stimulated oxidative damage and cell morphology, prevented ROS synthesis and lipid peroxidation, and enhanced antioxidant status | [51] |
| 38 | Ducrosia anethifolia | Wistar rats | Aerial parts (leaves and flowers) | Rats were administered with the EO of the species (500 mg/kg). Mortality and morbidity were analyzed. Pentylenetetrazole (PTZ, 80 mg/kg) was injected for stimulating convulsions in mice. Administration of rats 30 min before treatment with PTZ, diazepam (2 mg/kg), EO (25, 50, 100, and 200 mg/kg), and α-pinene (0.2 and 0.4 mg/kg) were supplemented. Mice behavior was recorded with a CD camera. | EO exhibited activity against PTZ- stimulated seizures, which can significantly decrease convulsing in rats. Death rate and PTZ-stimulated seizures decreased significantly after pretreatment with EO and α-pinene. EO and α-pinene were able to reduce oxidative stress features significantly after seizures stimulated by PTZ | [70] |
| 44 | Wistar rats | Rats were administered with varying doses (0.003%, 0.03%, and 0.3%) of α-pinene odor. Mice were remained in cages at a constant room temperature and were kept at 12 h dark and 12 h light condition with food and water. After being given the odor of varying doses of α-pinene, rats were exposed to different, unfamiliar environments | There was alteration in body temperature (abrupt increase) at 0.03% α-pinene after the transfer from home cage. However, 0.003% and 0.3% α-pinene odor decrease the stress stimulated hyperthermia in mice. 0.003% and 0.03% did not display any alteration in heart rate, but 0.3% led to changes. Varying doses of α-pinene bind to different olfactory receptors and stimulate different type of neuronal activities | [76] | ||
| α- and β-pinene | ||||||
| 39 | Sigma-Aldrich | Male Swiss Albino mice (Mus musculus) | - | Rats were treated with α- and β-pinene. Pretreated mice were supplemented with pentylenetetrazole (80 mg/kg i.p.) to induce seizures followed by one h of treatment. Mice were sacrificed by cervical dislocation, and brains, hippocampus, and striatum were removed immediately for neurochemical analysis | Significant seizure intensity reduction was observed at 400 mg/kg. Mixture of 400 mg/kg α- and β-pinene significantly enhanced the latency of the first convulsion. β-pinene and mixture (400 mg/kg) significantly enhanced the mortality time of rats. α-pinene and equimolar mixture remarkably decreases the hippocampal nitrite level and striatal content of dopamine and norepinephrine | [72] |
| S. No. | Source/Species | Compound | Model | Plant Portion | Method | Result | Ref |
|---|---|---|---|---|---|---|---|
| Respiratory system | |||||||
| 37 | - | α-pinene | BALB/c female mouse | - | α-pinene (0.1, 1, and 10 mg/kg) was administered to rats once a day for 10 days, 1 h before or 1 h after intranasal OVA challenge. HMC-1 cells were cultured into IMDM medium. Cell viability was assessed | Pretreatment with α-pinene reduced clinical symptoms, i.e., reduction in number of nasal, eye, and ear rubs and spleen weight; a decline in IL-4 levels; and a reduction in the level of nasal immunoglobulin E in OVA-induced rats | [69] |
| 48 | Fluka chemicals | α- and β-pinene enantiomers | OF1 (I.O.P.S. Caw) and KTL [(Hsd/Ola):NIH/(SPF)] male mice | - | Rats were placed in steel cages. Then, rats were kept in glass tubes (body plethysmograph). Rats were exposed (15 min) to selected pinene enantiomers. Differential pressure transducer attached with pneumotachograph was used to analyze inspiratory (VI) and expiratory (VE) air flow | Initially, no irritation was recorded in rats kept in room air. After the introduction of pinene enantiomers, the irritation was recorded, which indicates D-enantiomers were efficient sensory irritants. RD50 for pinene D-enantiomers was almost equal | [79] |
| 49 | Fluka chemicals | α-pinene enantiomers | BALB/c mice | - | Sensory irritation, airflow limitation, and pulmonary irritation of pinene have been studied | Sensory irritation was observed on the upper respiratory tract by (+) enantiomer during exposures 100 to 369 ppm. Initial dose was 70 ppm, which is nearest to the non-effective level (40 ppm) in humans. 200 ppm and higher concentrations triggered airflow limitations | [80] |
| 50 | Sigma-Aldrich | α-pinene | Human volunteers | - | Human volunteers were exposed in an exposure chamber for inhalation (2 h, 50 W) of α-pinene (10–450 mg/m3). After the exposure, capillary blood, urine, and exhaled air were determined | Absolute uptake of α-pinene enhanced linearly with exposure dose. α-pinene dose was firstly increased rapidly in arterial blood during the exposure, and then leveled off up to the end of exposure. Some undesirable effects were recorded during the exposure | [40] |
| Nephropathy | |||||||
| 51 | - | Piperazine ferulate tablets, + eucalyptol, limonene, and pinene soft capsules | Children with IgA nephropathy | - | Control group patients were administered with conventional or hormone therapy. Observation group patients were supplemented with piperazine ferulate tablets (0.1 g/dose and 3 times/day) coupled with eucalyptol, limonene, and pinene enteric soft capsules (0.1 g/dose and two times/day) for six months. | Effective rate of observational group (12 patients) was remarkably higher than hormone group (18 patients). Variations in serum IgA, fibronectin, and complement C3 of selected two groups were not statistically significant | [82] |
| Exposure | Uptake | Distribution | Elimination | |||
|---|---|---|---|---|---|---|
| Exhale Air | Blood | Urine | ||||
| Inhalation (8 volunteers, with light exercise-50W) [81,95,96] | ||||||
| α-pinene (+) | 2 h 450, 225, or 10 mg/m3 | Relative net uptake 59–62% * | tmax 120 min cmax 20 µMol/L(for 450 mg/m3) cmax 10 µMol/L(for 225 mg/m3) (exposure concentration depended) * cmax 10 µMol/L | 7.7% | cl21h 1.9 lkg−1h−1 | 0.001% In 30 min 4% of total uptake as cis and trans verbenol |
| t1/2 (3 phases α, β, γ) α-4.8 min β-38 min γ-695 min | ||||||
| α-pinene (−) | 450 mg/m3 | 7.5% | cl21h 1.16 lkg−1h−1 | |||
| t1/2 α-5.6 min β-40 min γ-555 h | ||||||
| β-pinene | 450 mg/m3 * | Relative net uptake 66% * | cmax 3 µMol/L * | * 5.7% | * cl21h 0.5 lkg−1h−1 * t1/2 α-5.3 min β-41 min γ-25 h | Not available |
| Dermal application in vitro [93], Ex vivo [92] | ||||||
| α-pinene | 1000 µL (concentration is not provided) for 27 h | Papp 6.49 × 10−5 cm/s | ||||
| 100 mg/cm2 applied on 0.65 cm2 at 37 °C ¥ | cmax 40 µg/cm2 tmax 15 min in SC | |||||
| β-pinene | 1000 µL (concentration is not provided) for 27 h | Papp 4.48 × 10−5 cm/s | ||||
| 100 mg/cm2 applied on 0.65 cm2 at 37 °C ¥ | cmax 290 µg/cm2 tmax 60 min in SC | |||||
| Oral administration (four volunteers) [89] | ||||||
| α-pinene | 9 mg (66 µmol) | Unmetabolized state—not detected (<4 µg/L) | t1/2 MYR-1.7 h tVER-1.0 h cVER-0.8 h | tmax 1.6 h (metabolites) | ||
| tmax 1–3 h Metabolites | t1/2 MYR-1.5 h cVER and tVER-1.6 h MYRA-1.4 h | |||||
| cmax MYR-11 µM tVER-26 µM cVER-9.3 µM | cl24h MYR-1.5%, cVER-5.6%, tVER-4.1% MYRA-6.7%. | |||||
| 78% unknown elimination, which could be exhalation or first-pass metabolism | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; L.D. Jayaweera, S.; A. Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. https://doi.org/10.3390/biom9110738
Salehi B, Upadhyay S, Erdogan Orhan I, Kumar Jugran A, L.D. Jayaweera S, A. Dias D, Sharopov F, Taheri Y, Martins N, Baghalpour N, et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules. 2019; 9(11):738. https://doi.org/10.3390/biom9110738
Chicago/Turabian StyleSalehi, Bahare, Shashi Upadhyay, Ilkay Erdogan Orhan, Arun Kumar Jugran, Sumali L.D. Jayaweera, Daniel A. Dias, Farukh Sharopov, Yasaman Taheri, Natália Martins, Navid Baghalpour, and et al. 2019. "Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature" Biomolecules 9, no. 11: 738. https://doi.org/10.3390/biom9110738
APA StyleSalehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., L.D. Jayaweera, S., A. Dias, D., Sharopov, F., Taheri, Y., Martins, N., Baghalpour, N., C. Cho, W., & Sharifi-Rad, J. (2019). Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules, 9(11), 738. https://doi.org/10.3390/biom9110738











